- Favipiravir, also known as T- 705, is an antiviral drug which is approved to treat pandemic influenza virus infections in Japan in 2014. It was discovered by Toyama chemical Co. Ltd. In Japan.
- Favipiravir is a pyrazine carboxamide derivative with activity against RNA viruses. It is a purine nucleic acid analogue.
- Its IUPAC name is 6-fluoro-3-hydroxypyrazine-2-carboxamide.
Mechanism of action of Favipiravir
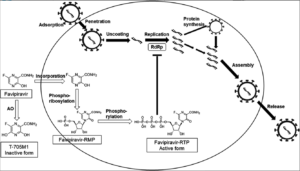
Figure 1- Mechanism of action of favipiravir (Source- Du et al, 2020)
- Favipiravir is a prodrug which is converted intracellularly into its active, phosphorylated form; favipiravir-RTP. HGPRT (Human hypoxanthine guanine phosphor-ribosyl transferase) play key role in this activation process.
- This active form is recognized as substrate by viral RNA-dependent RNA polymerase (RdRp) resulting in selective inhibition of viral RNA-dependent RNA polymerase. This ultimately prevent viral transcription and replication.
- Some research also suggest that it induces lethal RNA transversion mutation and produce nonviable viral phenotype.
- It has broad spectrum of activity against RNA viruses including influenza virus, rhino virus and respiratory syncytial virus. Favipiravir is not effective against DNA viruses.
- It doesn’t affect RNA and DNA synthesis in mammalian cells and hence is not toxic for them.
Therapeutic Use of Favipiravir
- Favipiravir is approved to treat influenza in Japan. However, it is indicated only for novel influenza rather than seasonal influenza. It is effective against all subtypes and strains of influenza viruses including ones sensitive or resistant to marketed neuraminidase and M2 inhibitors.
- It was stockpiled by Japanese government and Taiwanese center for Disease Control as a countermeasure for severe influenza.
Research on favipiravir
- During 2014 West Africa Ebola virus outbreak, some research performed on mouse models suggested that it may be effective against Ebola. It was effective in reducing mortality in patients with low to moderate level of Ebola virus, but not effective against high level of virus.
- It has been researched for use against Nipah virus, West Nile virus, yellow fever virus, foot and mouth disease virus, flaviviruses, arenaviruses and alphaviruses.
- Favipiravir has also showed effectiveness against Zika virus but was less potent than other drugs.
- It has been used as emergency and compassionate treatment for Lassa fever, norovirus and rabies cases.
Favipiravir and COVID-19
- An outbreak of 2019 novel corona virus infection named as COVID-19 has spread across the world. There is no specific antiviral drug approved for treatment of COVID-19.
- It is one of the experimental drugs (antiviral candidate) on trial for COVID-19. It has shown some promise in early trials in treatment of COVID-19.
- In early trials carried out in Shenzhen in February 2020, it showed a significantly faster mean time to viral clearance than lopinavir/ritonavir [4 days vs 11 days (P<0.001)]. These results were supported by an early Chinese randomized clinical trial (RCT), where favipiravir treatment results in greater recovery rate in non-critical COVID-19 patients than umefenovir (71.4% vs 55.9% [P<0.05]) [6]. Neither of them was effective in boosting recovery rate for critically ill patients in this same trial.
- It is currently being tested in 18 clinical trials for COVID-19 and results from two trials had shown positive results. And it has shown clinical improvements of up to 88 % in COVID-19 with rapid reduction in viral load by four days.
- It has been approved for the treatment of COVID-19 in China, Japan, Russia and Italy as of June 11, 2020.
Pharmacokinetics
- The maximum plasma concentration occurs 2 hours after oral administration and then decreases rapidly leading to short half-life of 2-5.5 hours.
- The plasma protein binding is 54%.
- It is metabolized by liver and excreted by kidneys.
Adverse Effects
- It is relatively safe and tolerable for short-term use. More study and evidence are needed to access its effect in long-term use.
- It causes increase in blood uric acid level by decreasing their excretion. The uric acid level comes to normal after discontinuation of favipiravir.
- It can cause vomiting, decreased body weight and decreased locomotive activity.
- It is teratogenic; when given to pregnant woman, can cause harm to fetus.
Drug Interactions
- It reduces metabolism of drugs like apomorphine, aminophenazone and reduces excretion of benzylpenicillin when administered together.
- It also interacts with hydroxychloroquine, ibuprofen, hydrocortisone, digoxin, diclofenac and fluorouracil.
Contraindication
- It is contraindicated in pregnancy as it causes birth defects and fetal death.
Reference
- Mishima E, anzai N, Miyazai M, abe T. Uric Acid Elevation by Favipiravir, an Antiviral Drug. The Tohoku Journal of Experimental Medicine. 2020; 251(2): 87-90.
- Delang L, Abdelnabi R, Neyts J. Favipiravir as a potential countermeasure against neglected and emerging RNA viruses. Antiviral Research. 2018; 153: 85-94.
- Pilkington V, Pepperrell T, Hill A. A review of the safety of favipiravir – a potential treatment in the COVID-19 pandemic? J Virus Erad. 2020 Apr; 6(2): 45–51.
- Furuta Y, Komeno T, Nakamura T. Favipiravir (T-705), a broad-spectrum inhibitor of viral RNA polymerase. Proc Jpn Acad Ser B Phys Biol Sci. 2017; 93(7): 449–463.
- Du YX, Chen XP. Favipiravir: Pharmacokinetics and Concerns About Clinical Trials for 2019-nCoV Infection. Clinical Pharmacology and Therapeutics. 2020; 0(0); 1-6.
- Shirakia K, Daikokub T. Favipiravir, an anti-influenza drug against life-threatening RNA virus infections. Pharmacol Ther. 2020; 209: 107512.